ProClinix works with your clinical trial team to deliver services related to patient recruitment and retention. We have over 20 years of experience working directly with sponsors and CROs on their trial needs and have worked with many types of trials including but not limited to endocrinology, neurology, respiratory, oncology, gastroenterology, and vaccines.
-
studyrequest@proclinixinc.com
Our Process
We have streamlined the process of working with our team to deliver efficiently and on time per your study timeline.

Building The Quote
A highly skilled research-experienced project manager is assigned as your point of contact throughout the entire time you are working with ProClinix. The project manager will spend time understanding your trial needs and quickly turn around a quote for you to share with your team and/or the sponsor.

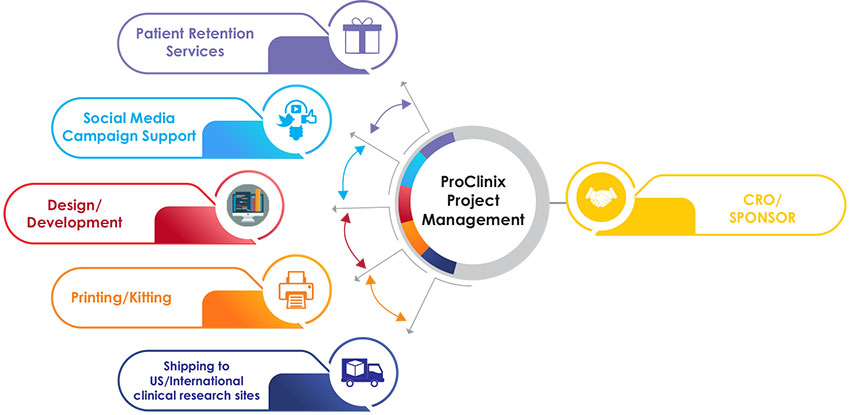
Design/Development
Once the quote is approved, we get started as soon as possible to draft designs for all the materials and/or place orders for retention items. Our experienced designers work to create drafts of designs for your study. The project manager will take care of all the communication between you and the designers to ensure the final version gets delivered according to the timeline.

Printing/Kitting
We work with a printing facility and ensure that kits are prepared for sites. At this point, along with the project manager you work with our study coordination assistant who will start tracking the orders as you request materials to be printed for sites. The study coordination assistant will communicate with the printing facility and ensure kits are prepared in time for the targeted shipping date.

Shipping
The project manager and study coordination assistant will work with our logistics partner to prepare the necessary shipping documents. ProClinix has many years of experience handling shipments both domestic and international.

